The Human Fertilisation and Embryology Authority (HFEA) is currently undertaking a review of the “law on fertility treatment regulation and embryo research”.
As part of its review, the HFEA has launched a consultation in which it makes a “case for change” to current laws, seeking to extend its powers and expand research using human embryos.
This could lead to the greatest changes to the law concerning the use of embryos since the 2008 Human Fertilisation and Embryology Act that allowed the creation of human-animal hybrid embryos and ‘saviour siblings’.
Removing the 14-day limit on experimenting on embryos
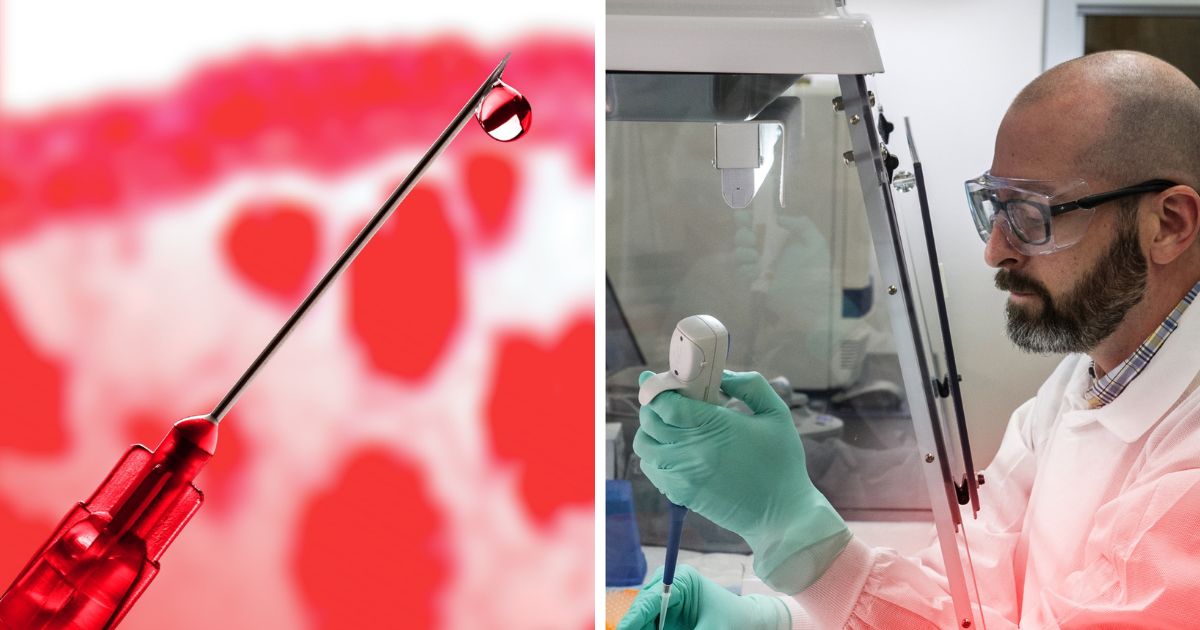
Currently, the Human Fertilisation and Embryology Act limits the use of human embryos in research to 14 days or the appearance of a primitive streak (if earlier).
In the consultation, the HFEA makes the case for removing the 14-day limit from current legislation, outlining the desirability of doubling the existing 14-day age limit during which research on human embryos is permitted. This would allow research on embryos that are four weeks old.
While in the past it was not technically feasible to culture human embryos beyond formation of a primitive streak or 14 days post-fertilisation, culture systems have evolved, now introducing this possibility.
Many countries such as Germany, Italy and Austria do not allow embryo research at all.
At around 22 days, the central nervous system begins to form and by 28 days, the heart has begun to beat, the brain has begun to develop and a baby’s eyes, ears and nose have started to emerge.
All major parties supported having a 14-day limit
In countries where embryo research is permitted, the 14-day limit has been adhered to across the globe and was upheld as a vital safeguard by each of the main UK political parties when the 2008 Human Fertilisation and Embryology Act was passed.
In the Parliamentary debate which preceded the Act becoming law, the Labour health secretary (Alan Johnson), Conservative shadow health secretary (Andrew Lansley), Liberal Democrat health spokesperson (Norman Lamb) and the Chair of the Health Select Committee (Kevin Barron) all referenced the 14-day limit as an important safeguard for embryo research.
Kevin Barron was even able to remark that “no one is suggesting that the 14-day limit on which we agreed in 1990 should be changed”. Significantly, Ken Clarke, who, as health secretary, had introduced the original Human Embryology and Fertilisation Act in 1990 that enshrined in law the 14-day limit, told the House of Commons “I believe that the judgement of 14 days or the emergence of the primitive streak has held up well for the past 18 years and we should stick to it”.
The importance of a 14-day limit was further underlined by Dawn Primarolo, a health minister, in her closing of the debate when she emphasised, with regard to human-animal embryos that were a central focus of the 2008 Bill, that “the Bill sets out strict prohibitions to guard against abuses. It makes it an offence to keep an admixed embryo beyond 14 days… Those are genuine safeguards”.
More recently, in 2017, Baroness Warnock, the Chair of the commission that set the original 14-day limit, warned of a slippery slope if the limit were to be removed.
Concerns about no time limit on experiments
As part of its case for making this change, the HFEA mentions that the International Society for Stem Cell Research recently proposed new guidelines to remove the 14-day limit on embryo research. These guidelines recommend removing the 14-day limit and do not mention any gestational limit for research on embryos.
Bioethicists are concerned that the guidelines include no such gestational limit.
Hank Greely, a Stanford University bioethicist, commented “If you don’t have any endpoint, could you take embryos to 20 weeks? To 24 weeks? Is viability the only endpoint? Is viability even an endpoint?”
Questions regarding HFEA independence and impartiality
The HFEA has previously been criticised regarding both its independence, as a regulator, and its impartiality in relation to the ethical standards it is obliged to uphold, which include Parliament’s insistence on the “special status” of the human embryo.
As far back as 2002, Suzi Leather, then Chair of the HFEA, told the US President’s Council on Bioethics in Washington D.C. that the HFEA committee would not include members who oppose human embryo research as their views “are outwith the moral consensus in the UK”.
In 2005, the House of Commons Science and Technology Committee expressed “concerns… that the HFEA has been campaigning, corporately, for changes in legislation”. It explained, “We are concerned that the HFEA has crossed the boundary from regulation to advocacy”.
More recently, in December 2021, the current Chair of the HFEA, Julia Chain, promised “We need a modernised law and during my tenure as Chair, this will be one of my main priorities”, seemingly treating the HFEA as a vehicle for campaigning for legislative change.
The HFEA is a regulator set up to implement the law as passed by Parliament. Its responsibility is to regulate the law not lobby to change it. Yet, in May 2022, in an interview with Peter Thompson, the chief executive of the HFEA, the Guardian reported that “the HFEA is seeking far-reaching changes” to the Human Fertilisation and Embryology Act. The Guardian even reported that the HFEA “is planning to propose draft legislation”.
Leading Canadian bioethicist Francoise Baylis criticised a previous HFEA consultation process for being “an exercise in strategic public relations”. The current consultation takes a similar approach, making the case for far-reaching changes without giving the other side of the argument and leaving the most controversial proposals to the end of its lengthy survey. Rather than offering protections for human embryos, whose special status is nowhere mentioned in the proposals, the consultation focuses instead on research and a desire for “innovation”.
Decreasing Parliamentary scrutiny of future changes
The HFEA also outlines that it wants to “future proof” the HFE Act to allow it more easily to be modified to permit changes, such as increasing or removing the 14-day limit, via secondary legislation rather than changes to primary legislation.
This would see future changes to embryology laws being demoted to secondary legislation, where scrutiny would be reduced, rather than being introduced through primary legislation, where ethical considerations can be rigorously discussed in Parliament and MPs held accountable.
Right To Life UK is calling on members of the public to make a submission to the consultation ahead of the closing deadline at 5pm on 14 April 2023. The public can do this through the HFEA consultation website here.
Regulator appears to have become lobbyist for industry it regulates
Catherine Robinson, spokesperson for Right To Life UK, said “The HFEA isa taxpayer-funded body that is supposed to exist to regulate experimentation on embryos. Instead, it appears to have taken on the role of lobbyist for the industry it is meant to be regulating, by making the case for removing the 14-day limit from current legislation.
“What we are talking about here is doing medical experiments on unique human embryos. Human embryos should never be experimented on, but it is even more disturbing to see the HFEA make the case for doing experiments on them even further into their development.
“At around 22 days, the central nervous system is formed and by 28 days, the developing heart can sometimes be seen beating, the brain has begun to develop and a baby’s eyes, ears and nose have begun to emerge.
“We are calling on members of the public around the UK to submit to the consultation ahead of the closing deadline at 5pm on 14 April 2023”.